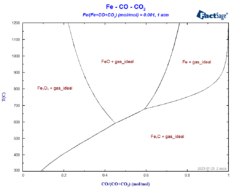
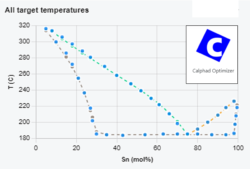
FactSage online training course
From July 8th to July 11th 2024 we will host a four-day online course on Computational Thermochemistry and the FactSage software. On each day, the course will take half a day, from 1 pm to 5 pm Central European Time (CET). To participants who don’t already have FactSage installed on their PCs we will make […]
FactSage online training course Read More »