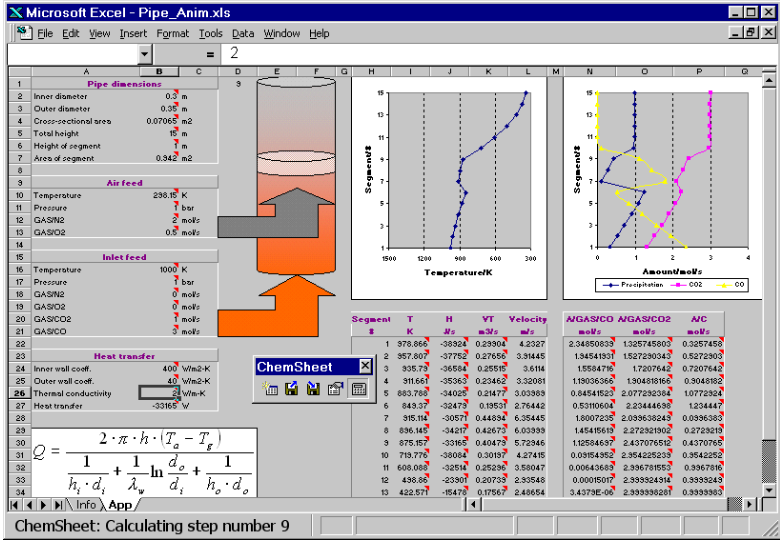
After the combustion and soot formation application, here, thermochemical calculations are linked with a physical heat transfer model. Similar to the previous application, CO2 and CO are intruduced as one stream where the individual amounts can be varied in cells B20 and B21. This stream is let in at the buttom of a 15 segments (1 segment = 1 m) long pipe, while air is let in as second stream at segment 7. Heat is lost by transfer from the gas to the wall of the pipe, transfer through the pipe and heat transfer from the pipe to the environment and the thermal conductivity (B26) is varied in two different calculations. Temperature an composition of the gas (CO and CO2 content) as well as the amount of soot formation are plotted in the diagrams.
Another example where thermochemical calculations were not only linked to heat transfer models but also to reaction kinetics is the titanium(IV)chloride burner, known for its wide industrial use in TiO2-pigment manufacture.
