In recent years, there has been a continuous societal and industrial effort to promote the so-called „circular economy“. According to this paradigm, for producing engineering materials in general, and metals and alloys in particular, the chemical elements which are presently necessary in our economy should not be extracted from the so-called primary ores but instead of this, one should aim at recuperating these elements, as much as possible, from scrap materials or from either industrial or residential waste and residues. The objectives of this approach are two-fold: by proceeding in this way, one would avoid unnecessary immediate material and energetic costs and, most important, one would reduce the environmental impact of the corresponding industrial activities, what would lead to an even more important cost reduction for future generations. The „circular economy“ concept is directly related to the concept of „urban mining“, whereby rare metals are extracted from waste electrical and electronic equipment (WEEE), bringing them back into the cycle.
The Ausmelt TSL process is presently one of the most important processes in non-ferrous extractive metallurgy, possessing a remarkable environmental performance and being both flexible and efficient. It may be used to extract metals both from primary and secondary sources and it was proven to be able to successfully extract Cu, Sn, Zn, Pb and Ni from its corresponding ores.
Among the many non-ferrous metals, the commodity metal lead occupies a special place by being presently one of the most recycled metals in the industry. In addition, lead may be utilized as a carrier platform for the recycling of many other metals [1].
Recently, a Simusage model for the oxidative stage of the TSL process for lead smelting was developed in the scope of the QuaResPro project, the source is available in GitHub [2]. The Simusage model is constructed by using a flowsheeting approach in which the reactor is subdivided in different interconnected zones, each one being in a state of local equilibrium. The natural flow direction of the materials both in the reactor and in the flowsheet corresponds to the downward flow of the condensed phases; the upward flow of the gases being realized by using the so-called iterators, a SimuSage artifact. In the process stage being simulated, the sulfidic concentrate should be progressively converted into a combination of two phases: a PbO-rich slag phase and a Pb-rich bullion.
In what follows, we highlight some of the most important results of the corresponding recent article by Rezende et al. [3]. In Figure 1, we show how the phase amounts after a 5h process time vary with the oxidizing gas amount for a concentrate containing 71.8 wt% of PbS. For a process gas flow rate between 216.2 and 220.7 kg/min, we would have optimal conditions, once one would avoid matte phase formation while and, at the same time, the amount of bullion would be above 92% of its maximum value (76.6 t) and its sulphur impurity content would stay below 500 ppm.
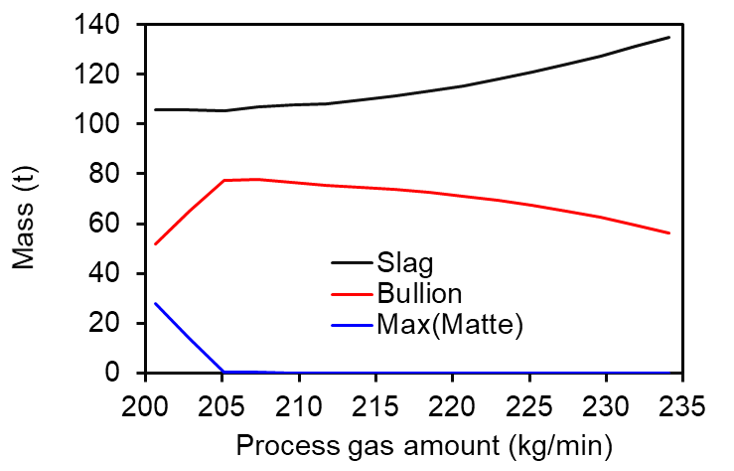
Figure 1
In Figure 2, we show how the oxygen partial pressure below the lance varies with process time, for four values of the process gas flow rate that were investigated. After an initial transient time of ca. 1h, during which the p(O2) significantly increases, it then decreases during the next 1h, the total time of 2h agreeing well with the corresponding total start-up time in the industrial practice. The level of p(O2) attained in steady-state in the case of a process gas flow rate equal to e.g. 222.9 kg/min compares well with the expected values for a stable process control, that should be between 10-6.5 and 10-7.5 atm.
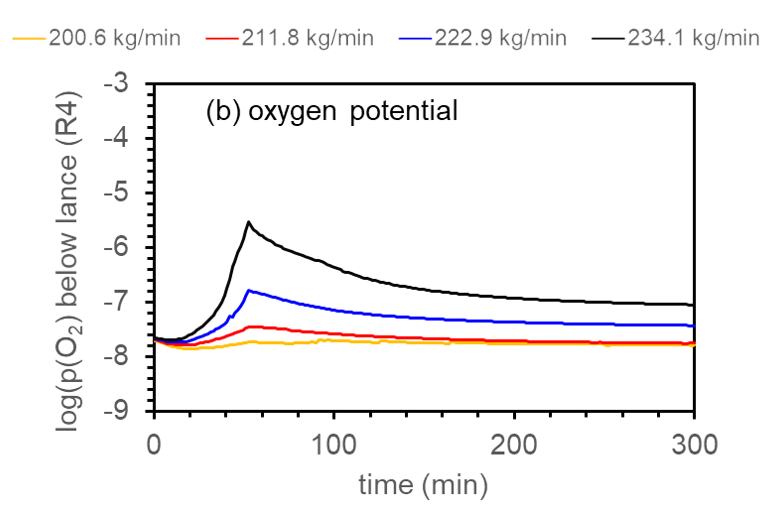
Figure 2
In Figure 3, we compare the curves for the steady-state oxygen partial pressure below the lance as a function of the process gas amount for two situations: for the original concentrate of Figure 1 and for a low quality concentrate containing only 68.2 wt% PbS. In spite of the shift in the process gas flow rate, the optimal oxygen partial pressure stays in the same range for both cases, as denoted by the bold lines showing the desired operation range, in which there is a compromise between maximizing bullion production and minimizing matte phase formation. Such valuable insight for different operation conditions is what makes SimuSage such a powerful tool for process modeling and the circular economy.
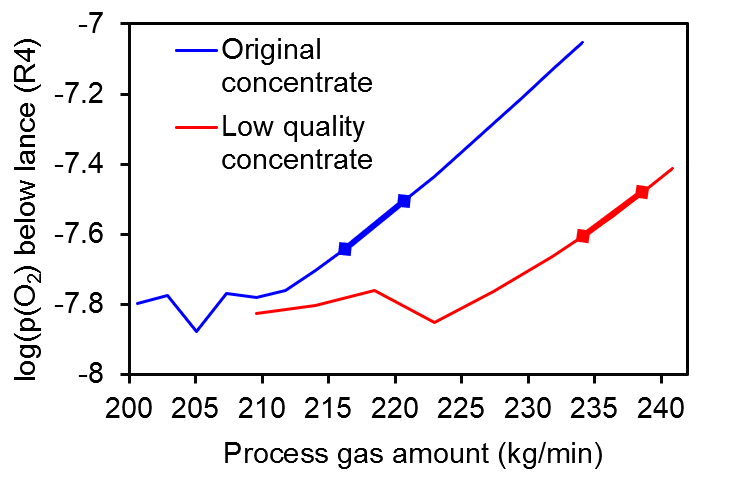
Figure 3
If you have further questions about thermochemically-based process modelling and Simusage, please contact us!
References
[1] van Schalkwyk RF, Reuter MA, Gutzmer J, Stelter M (2018) Challenges of digitalizing the circular economy: Assessment of the state-of-the-art of metallurgical carrier metal platform for lead and its associated technology elements. Journal of Cleaner Production 186: 585-601.
[2] The GitHub repository: https://github.com/GTT-Technologies/SimuSage-Lead-TSL-Oxidation
[3] Rezende J, van Schalkwyk RF, Reuter MA, to Baben M (2021) A Dynamic Thermochemistry-Based Process Model for Lead Smelting in the TSL Process. J. Sustain. Metall. 7: 964–977. https://doi.org/10.1007/s40831-021-00387-7
