The GTOx oxide database was developed in-house at GTT-Technologies and in close cooperation with Forschungszentrum Jülich, IEK-2, specially with Elena Yazhenskikh. The thorough literature review was performed concerning phase diagram information and thermochemical experiments (enthalpies of formation and transformation, component activities, oxygen partial pressures etc.). GTOx includes a description for different combinations of oxides, e.g. high Na2O- and K2O-containing systems like biomasses (2016-2019) or Vanadium oxide containing systems (2019-).
The present GTOx database (version 17) contains thermodynamic data covering binary, ternary and quaternary sub-systems among the following components:
Al2O3-Al2S3-CaO-CaF2-CaS-Cr2O3-CrS-CuO-Cu2O-FeO-Fe2O3-FeS-Li2O-MgO-MgS-MnO-Mn2O3-MnS-K2O-K2S-Na2O-Na2S-NiO-NiS-P2O5-SO3-SiO2-SrO-TiO2-Ti2O3-V2O3-V2O5-ZnO-ZnS
The phase list of GTOx comprises Gas, Slag, Liquid phase, 153 solid solution phases and 821 stoichiometric phases, which are required for the description of the systems from 20 basic (component) oxides. The database includes the thermodynamic descriptions of 252 binary, 189 ternary and 10 quaternary systems. Check our database documentation for more details (https://gtt-technologies.de/data/#gtox-gtt-oxides-database).
In the following, recent extension on the GTOx database are presented in more detail:
There has been an increasing interest in lithium (Li) and in its metallurgy as well as in its recycling because of its application as a material for rechargeable batteries. Lithium oxide (Li2O) has so far been integrated into the reduced core system CaO‑MgO-Al2O3-FeO-Fe2O3-MgO-MnO-Mn2O3-Na2O-NiO-SiO2-TiO2-P2O5-ZnO. This resulted in the thermodynamic evaluation of 13 binary, 23 ternary and 1 quaternary system based on the presently available experimental data.
Lithium was also introduced into the thermodynamic description of solid solution phases such as MeO, Spinel, Ca2SiO4-α, Ca2SiO4-α’, beta-prime and beta-alumina using available experimental information. In the lithium oxide containing systems particular attention was given to the phase Spinel which forms the wide completely miscible solid solution Fe3O4-LiFe5O8-LiAl5O8-MgAl2O4.
The following 11 new systems containing Li2O are assessed and added to the GTOx database:
FeO-Li2O-P2O5, Fe2O3-Li2O-P2O5, K2O-Li2O-P2O5, Li2O-FeO-SiO2, Li2O-Fe2O3-SiO2, Li2O-MgO-P2O5, Li2O-MnO-P2O5, Li2O-NiO-P2O5, Li2O-P2O5-SiO2, Li2O-P2O5-TiO2, Li2O-P2O5-ZnO.
Furthermore, in the extension of the database, 11 ternary combinations containing phosphorus pentoxide (P2O5) are considered. The following new systems containing P2O5 are assessed and added to the GTOx database:
Al2O3-K2O-P2O5, Al2O3-P2O5-SrO, CaO-P2O5-SrO, FeO-MgO-P2O5, FeO-P2O5-SrO, K2O-P2O5-TiO2, K2O-P2O5-V2O5, MgO-P2O5-SrO, P2O5-SrO-ZnO, P2O5-V2O5, P2O5-V2O5-ZnO.
The additional ternary systems containing Li2O and P2O5 have been thermodynamically assessed using all available experimental data. The experimental information on the thermodynamic properties of the systems such as phase diagram data, phase transition, heat of formation, are used for the generation of Gibbs energy datasets for all solid solution phases and compounds that were confirmed experimentally. As examples of such thermodynamic evaluations, in the following the systems K2O-Al2O3-P2O5 andLi2O-P2O5-ZnO are shortly presented.
The system K2O- Al2O3– P2O5
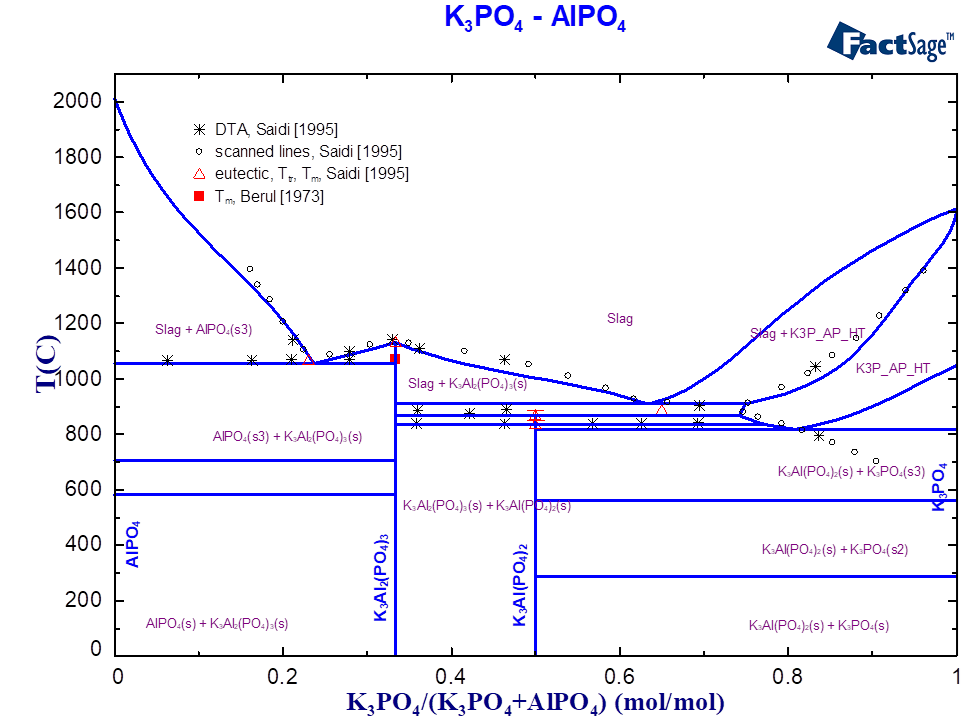
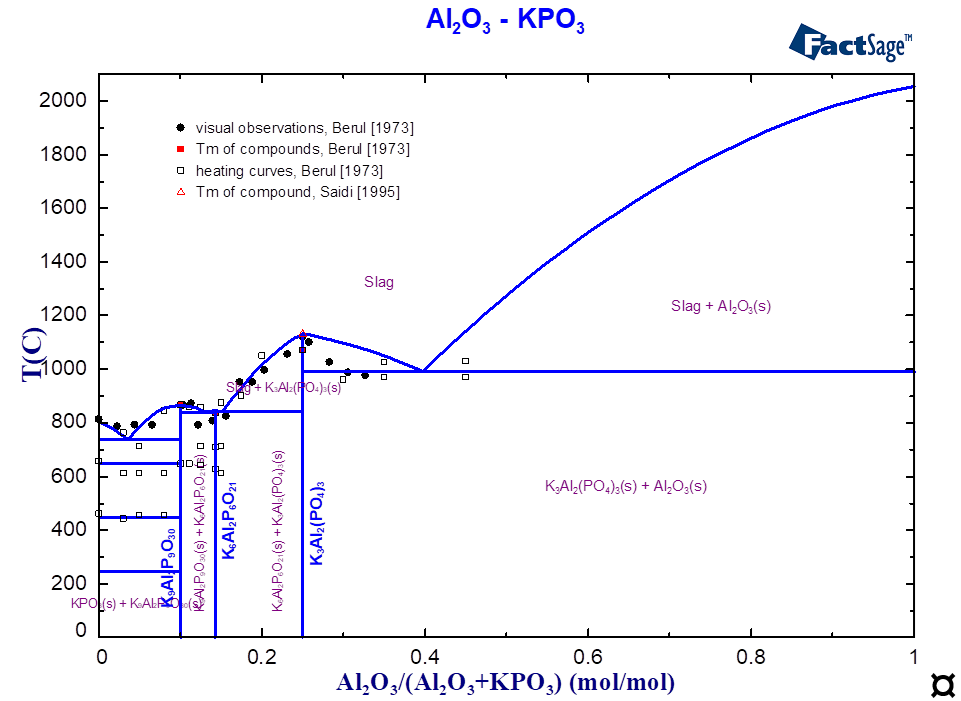
For the ternary system K2O-Al2O3-P2O5, only scarce experimental information (thermal behaviour, structure) was found in the literature [Berul1973, Saidi1995]. The existence of four stoichiometric compounds was reported (Table 1).
Table 1 – Stoichiometric compounds in the system K2O-Al2O3-P2O5
| Mole ratio K2O:Al2O3: P2O5 | Phase | Experiment, Tm, °C | Tm, °C (calc.) | Remarks |
| 3:1:2 | K3Al(PO4)2 | 867 [Saidi1995] | 867 | LT, HT phases |
| 3:2:3 | K3Al2(PO4)3 | 1070 [Berul1973], 1130 [ Saidi1995] | 1130 | in the slag: K3Al2(PO4)3/4 |
| 3:1:3 | K6Al2P6O21 | 836 [ Berul1973] | 844 | |
| 9:2:9 | K9Al2P9O30 | 865 [ Berul1973] | 864 | |
| 6:1:3 | K6Al(PO4)3 | virtual compound | — | solid solubility with K3PO4 |
Since thermodynamic data on ternary compounds do not exist in the commercially available databases, their Gibbs energy functions were constructed from those for pure oxides and optimised according to the phase diagram data. The ternary compound with the ratio K2O: Al2O3: P2O5=3:2:3 was taken as a ternary associate species in the slag so that the equilibrium between solid and liquid phases can be described correctly.
The solubility in the solid state is reported for the quasi-binary system K3PO4-AlPO4 [2]. The maximal solubility AlPO4 in K3PO4 is 25 mol.% at the temperature about 890 °C. Previously, the solid solutions based on corresponding crystallographic modifications of alkali orthophosphates, Alk3PO4 (Alk=Na, K), have already been introduced in the GTOx database for the systems containing Na2O, K2O, CaO, MgO and ZnO. Therefore, the solubility between K3PO4 and AlPO4 is embedded into the same solution phase. The complete formula of the solution with the Al2O3-containing component can be written as follow:
(K2O)2(P2O5)1(K2O, CaO, K2CaO2, MgO, K2MgO2, Na2O, K2AlPO5)1
The component with the composition K6Al(PO4)3 or 6:1:3 (Table 1) corresponds to the virtual compound on the section AlPO4-K3PO4 (Fig. 1), whose thermodynamic data have been optimised. The interaction between the components K2O and K2AlPO5 on the third sublattice describes the limit of solubility and the correct equilibrium with the slag. Figures 1 and 2 show the calculated phase diagrams of the K3PO4-AlPO4 and KPO3-Al2O3 systems. The equilibria between slags and solid phases are described using the optimised thermodynamic data on ternary compounds and the interactions in the solid and liquid solutions. The melting temperatures of all compounds are correctly reproduced (Table 1, Fig. 1-2).
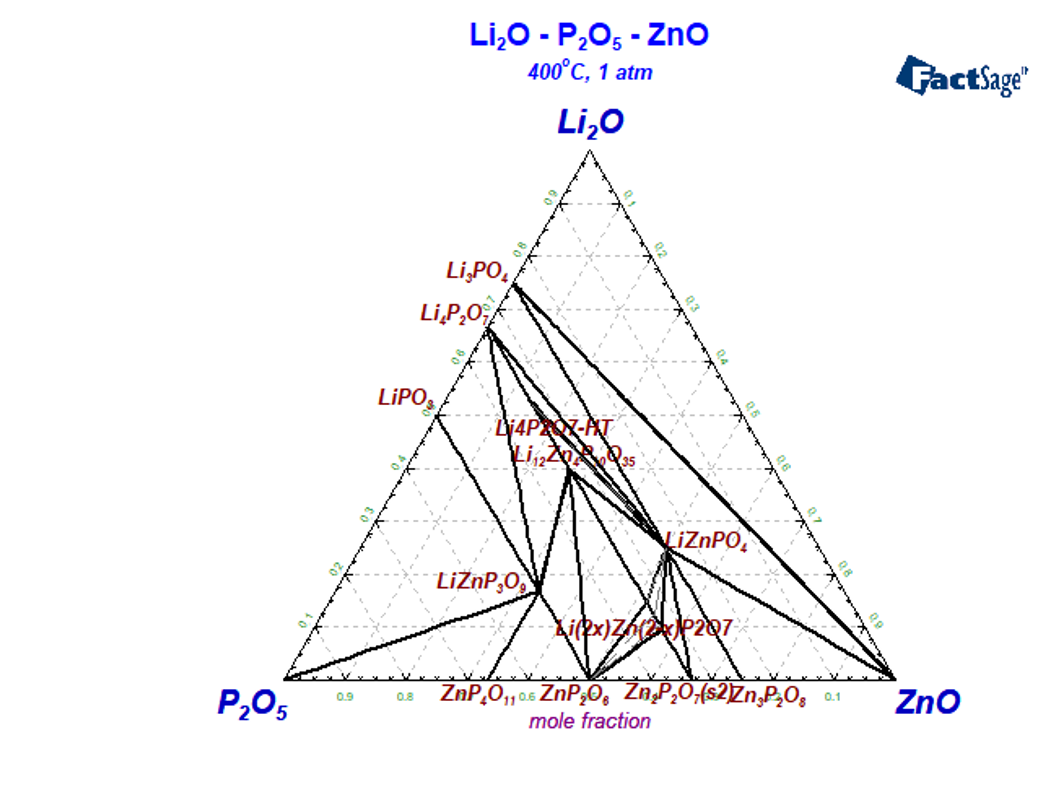
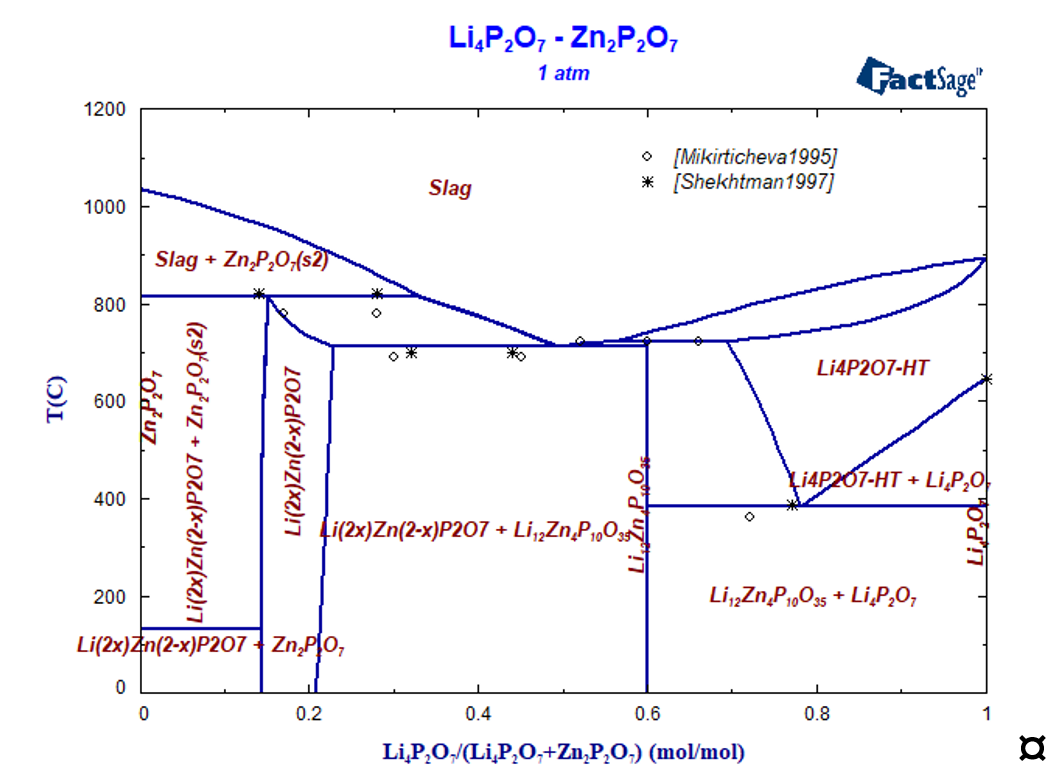
The system Li2O-P2O5-ZnO
In the Li2O-P2O5-ZnO system three ternary stochiometric phases LiZnPO4,Li12Zn4P10O35,LiZnP3O9 have been found. The system is characterized also by the presence of a relatively wide solid solution phase based on the composition 0.8Zn2P2O7.0.2Li4P2O7 and significant solubility of ZnO in Li4P2O7-HT. The melting temperatures and description of the ternary phases are summarized in Table 2.
Table 2 – Ternary phases in Li2O-P2O5-ZnO system
| Mole ratio Li2O:ZnO:P2O5 | Phase | Description | Experiment, Tm, °C | This work |
| 1:2:1 | LiZnPO4 | stoichiometric | 1190 [Ji2009] | 1167 |
| 6:4:5 | Li12Zn4P10O35 | stoichiometric | 720 [Petrova2007] 720 [Mikirtcheva1995] | 722 |
| 1:2:3 | LiZnP3O9 | stoichiometric | 678 [Averbuch1970] | 678 |
| Li(2x)Zn(2-x)P2O7 | (Li4P2O7, Li4Zn12P14O49) | 780 [Petrova2007] 820 [Shekhtman1997] 780 [Mikirtcheva1995] 766 [Ji2009] | 814 |
Isothermal section at 400 °C is presented in Figure 3, where all ternary stoichiometric and one solid solution phases are present. The isopleth section Li4P2O7-Zn2P2O7 was experimentally investigated by many authors using different methods [Mikirtcheva1995, Shekhtman1997, Petrov2000, Petrova2007], where the stoichiometric compound Li12Zn4(P2O7)5 and the solid solution phase berthollide Li2xZn2–xP2O7 melting peritectically were carried out. The phase diagram of the Zn2P2O7-Li4P2O7 polythermal section is characterized by both peritectic and eutectic invariant equilibria. The solid solution phase extends from 15 to 28 mol.% Li4P2O7· [Shekhtman1997, Petrov2000, Petrova2007] and melts incongruently. Instead of solid solution phase the stoichiometric compound Li6Zn(P2O7)2 is reported in [Ji2009] what is in contradiction with other experimental data.
Zinc oxide was introduced into the thermodynamic description of the Li4P2O7-HT phase to describe the experimental determined solubility of ZnO. Calculated isopleth section Li4P2O7-Zn2P2O7 is shown in Figure 4 compared with experimental data, the agreement is satisfactorily.
References
[Averbuch1970] M. T. Averbuch-Pouchot, M. A. Rakotomahanina -Rolaisoa, Bull. Soc. Fr. Mineral. Cristallogr., 93 [3] 394-396 (1970).
[Berul1973] S.I. Berul`, N.I. Grishina, Interaction of KPO3 with Al2O3, Zh. Neorg. Khim. 18(9) (1973) 2518-2522.
[Mikirticheva1995] G. A. Mikirticheva, V. I. Shitova, M. A. Petrova, A. S. Novikova, S. K. Kuchaeva, L. Yu. Grabovenko, R. G. Grebenshchikov, Zh. Prikl. Khim. (S.-Peterburg), 68 [3] 462-467 (1995).
[Saidi1995] M. Saidi, G. Coffy, F. Sibieude, Les systemes binares AlPO4-M3PO4 (M=Li, Na, K), J. Therm. Analysis 44 (1995) 15-23.
[Shekhtman1997] G. Sh. Shekhtman, E. I. Burmakin, and E. S. Korovenkova, Elektrokhimiya, 33 [5] 507-511 (1997); Russ. J. Electrochem. (Engl. Transl.), 33 [5] 552-556 (1997).
[Petrova2007] M. A. Petrova, G. A. Mikirticheva, R. G. Grebenshchikov, Neorg. Mater., 43 [9] 1141-1148 (2007).
[Ji2009] L. N. Ji, J. B. Li, Y. Q. Chen, J. Luo, J. K. Liang, and G. H. Rao, J. Alloys Compd., 486 [1-2] 352-356 (2009).
