Sometime ago a customer asked me how to find out if Ca2SiO4 is included in a certain database. Since the answer to this question can be really useful for customers in general, I decided to write this blog on the subject. We will define a substance with a defined chemical composition as a chemical species. In this particular case, we are interested in finding the pure solid species Ca2SiO4(s) in a certain database.
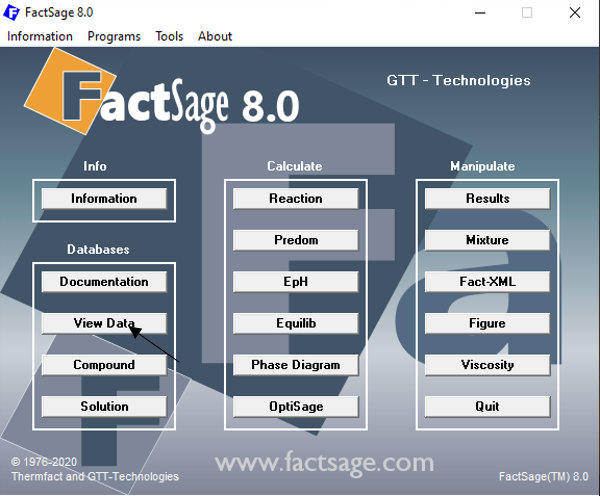
Figure 1
We start by clicking on the button “View Data” on the FactSage main window (Figure 1). Then, on the screen area “Data”, we click on the “compound” radio button (Figure 2). This means that we are searching for Ca2SiO4 as a pure substance. On the drop-down Menu for the selection of the databases, we choose “All Databases” (Figure 2). Finally, in the input field at the bottom, we enter the chemical formula Ca2SiO4.
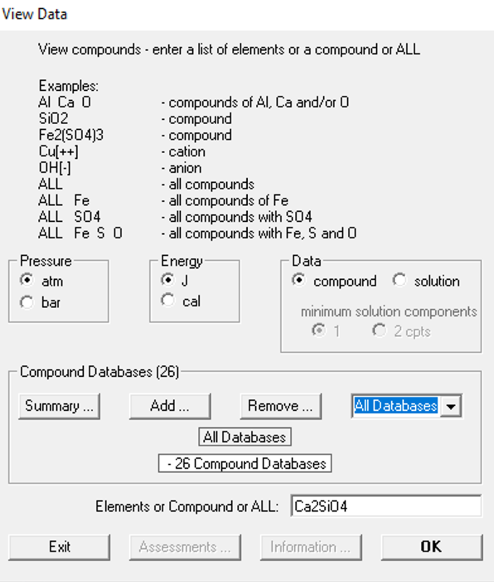
Figure 2
In Figure 3, we see that Ca2SiO4 was found, in the FTdemo database. Maybe this species was found in other databases also? Let us check it out.
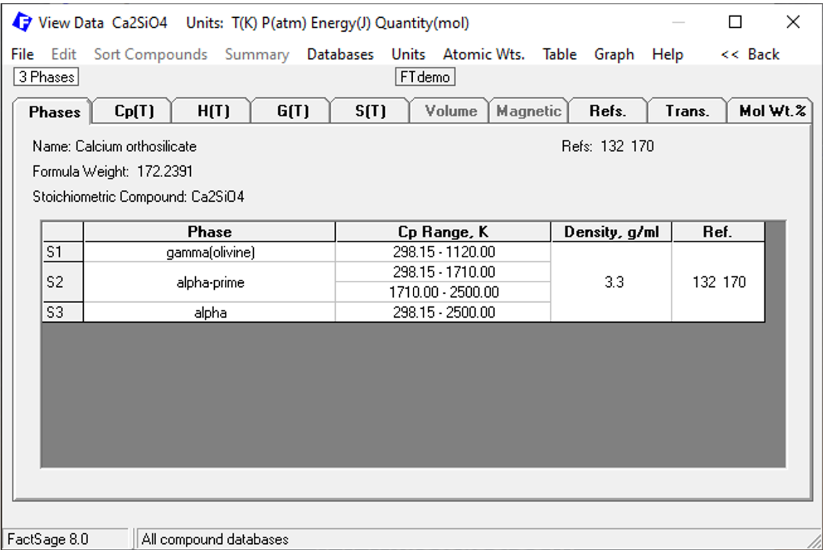
Figure 3
We click on the upper Menu bar at “Databases”. We verify that Ca2SiO4 is present in the highlighted databases: FTdemo, SGPS, FactPS, FThelg, FToxid and GTOx. The databases that will be highlighted depend on your specific installation of FactSage and on the corresponding installed databases. Let us click on FToxid (Figure 4).
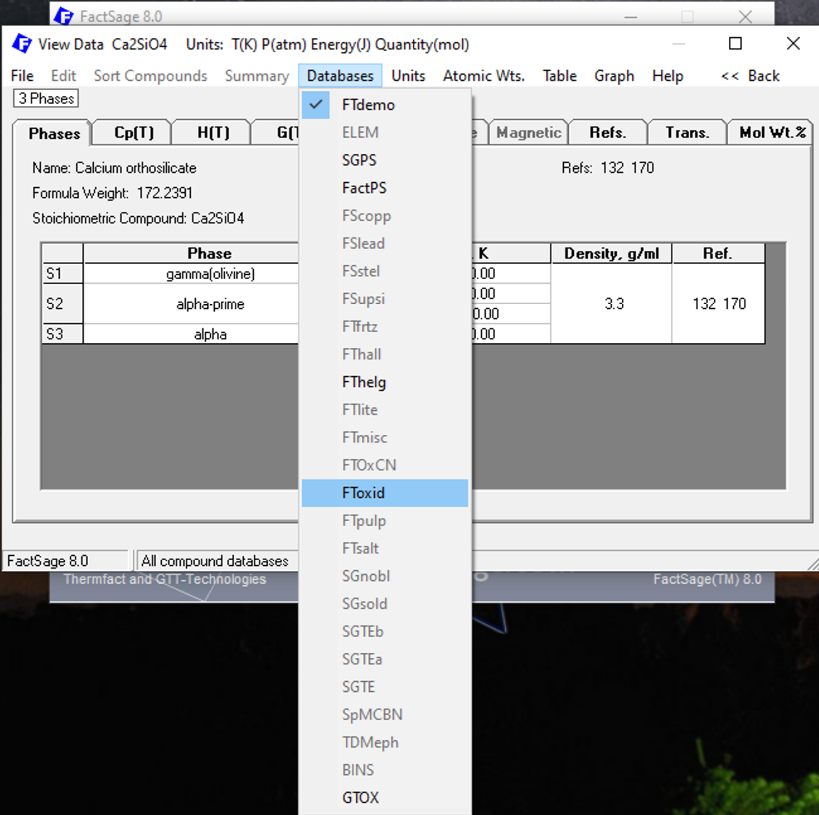
Figure 4
In Figure 5, we see that Ca2SiO4 is present in FToxid in three possible solid phases: S1: gamma olivine, S2: alpha-prime, S3: alpha.
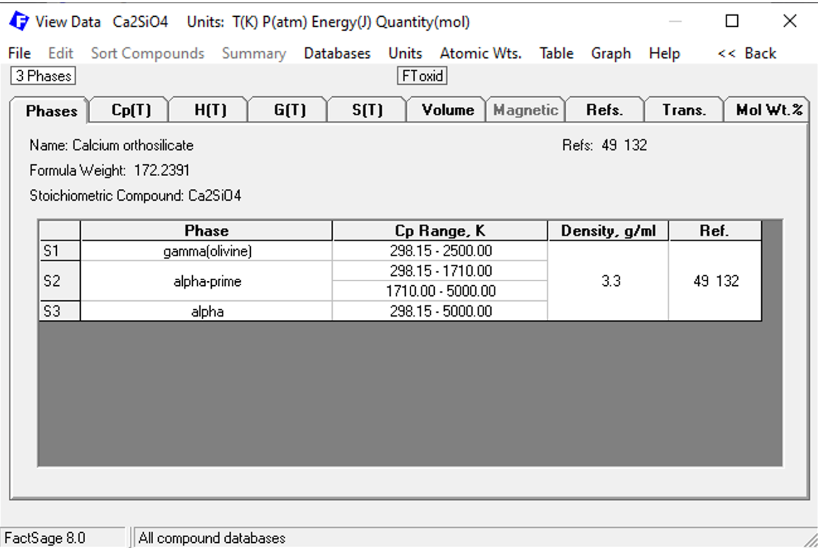
Figure 5
Now there is an important point: Ca2SiO4 may also be present inside a solid solution phase. A solid solution phase has a variable chemical composition and it may be that Ca2SiO4 is described by the corresponding solution model at some extreme value of the elemental compositions for the solution. We say that Ca2SiO4 is an end-member of the corresponding solution. To check for this, we close the present window and, by doing so we come back to the previous one. Then, on the window that appears, we select the “solution” radio-button instead of the “compound” radio button (Figure 6). Notice that now, we are obliged to select a specific database: we select “FToxid” on the drop-down Menu (Figure 6). The chemical formula is maintained: Ca2SiO4.
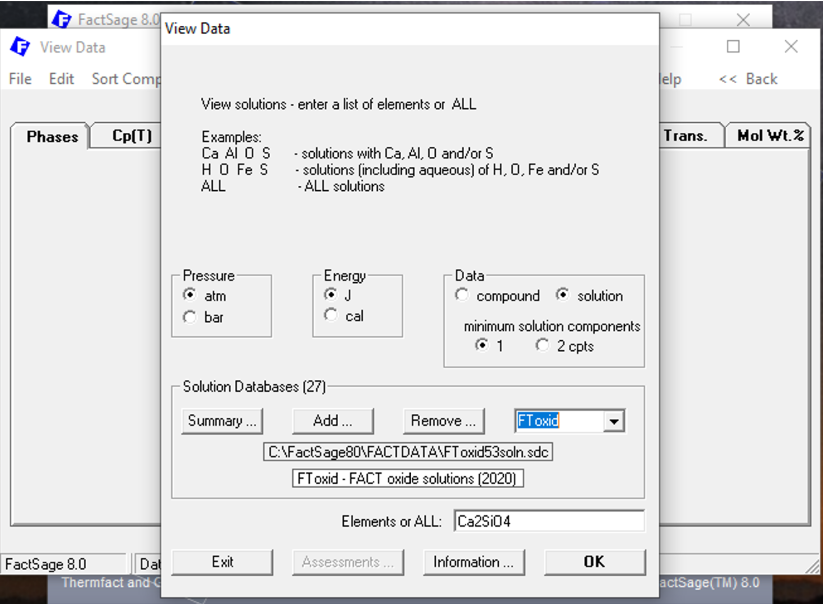
Figure 6
When we then click on “OK”, Figure 7 appears. Notice that the window shown on Figure 7 exhibits phases in the FToxid database containing two or more of the elements Ca, Si, O. I have marked within a red square the phases containing specifically Ca2SiO4 as an end-member, these are: FToxid-bC2SA, FToxid-aC2SA and FToxid-OlivA.
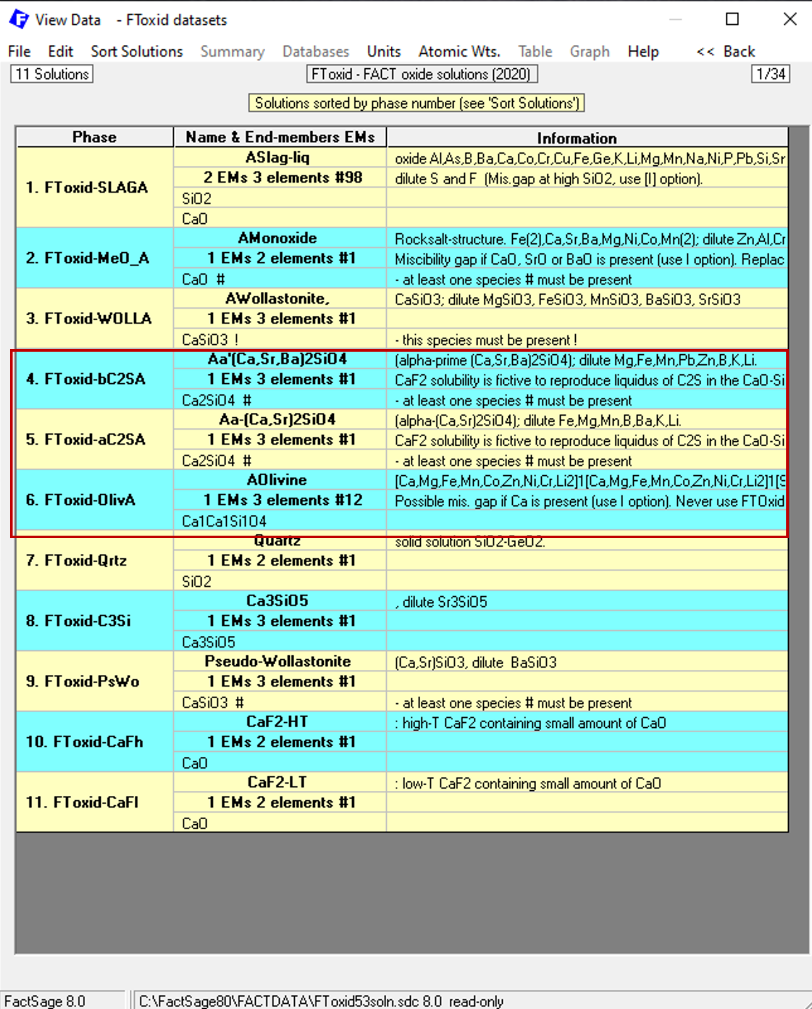
Figure 7
There is another way of searching for the Ca2SiO4 species, which I think has some advantages over using the “View Data” module. We start all over again on the FactSage main window, but now we click on the button “Documentation” (Figure 8).
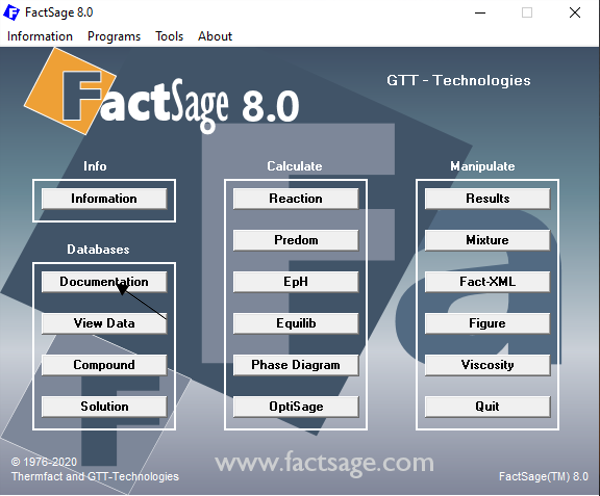
Figure 8
On the window which opens up, we click on “description of solutions”, under the FToxid node (Figure 9).
Figure 9
Now a window pops up with all the solutions contained in FToxid (Figure 10). To search now for “Ca2SiO4“, we press Ctrl-F and enter “Ca2SiO4” into the input field of the window which pops up (Figure11). The program will then highlight all the appearances of Ca2SiO4 in the file (Figure11). Notice that, in this case, we find the exact species that we were searching for. In addition to this, this file contains more contextual information about the solution phases containing this species as a constituent end-member than it was seen by using “View Data”.
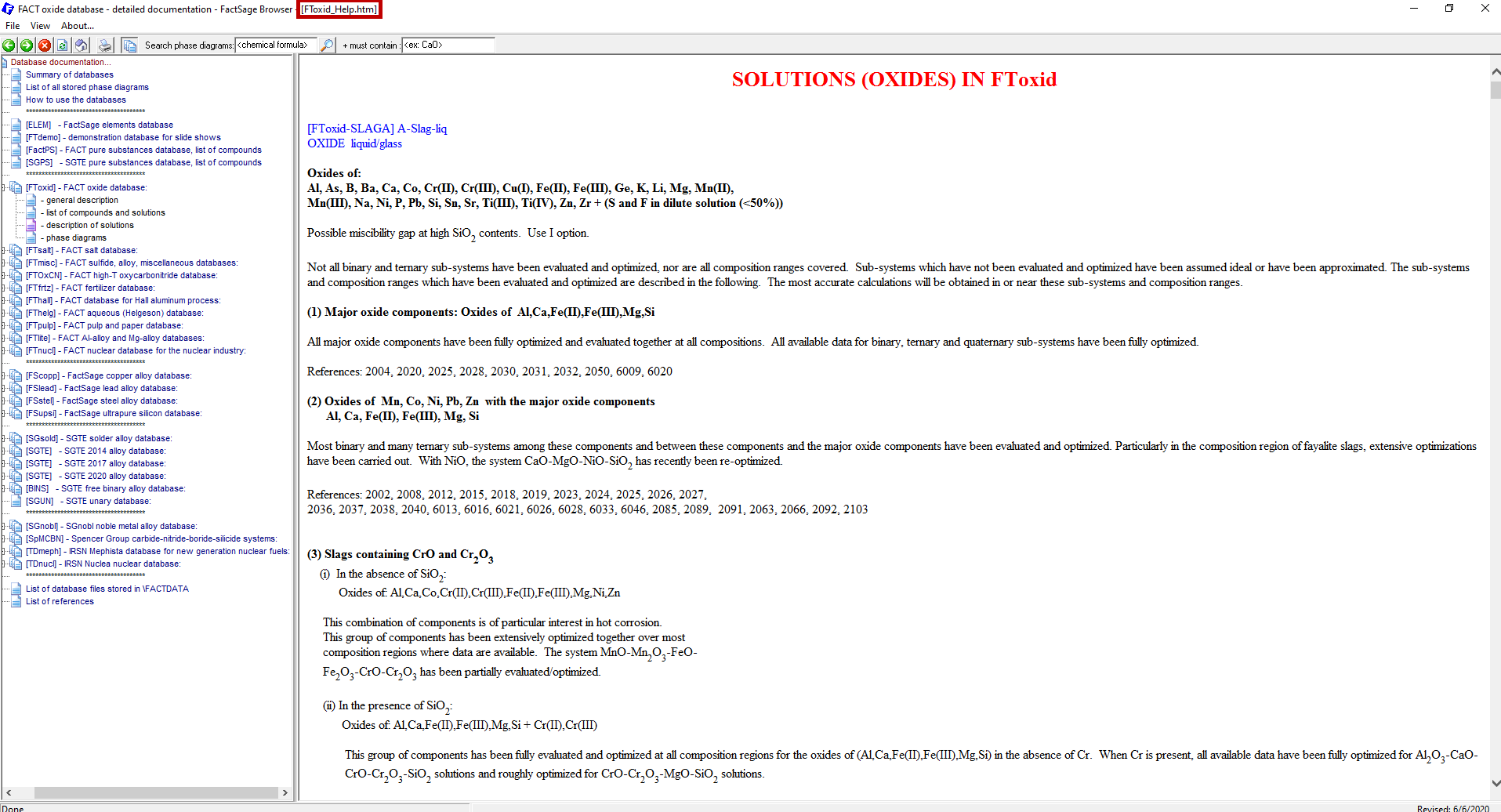
Figure 10
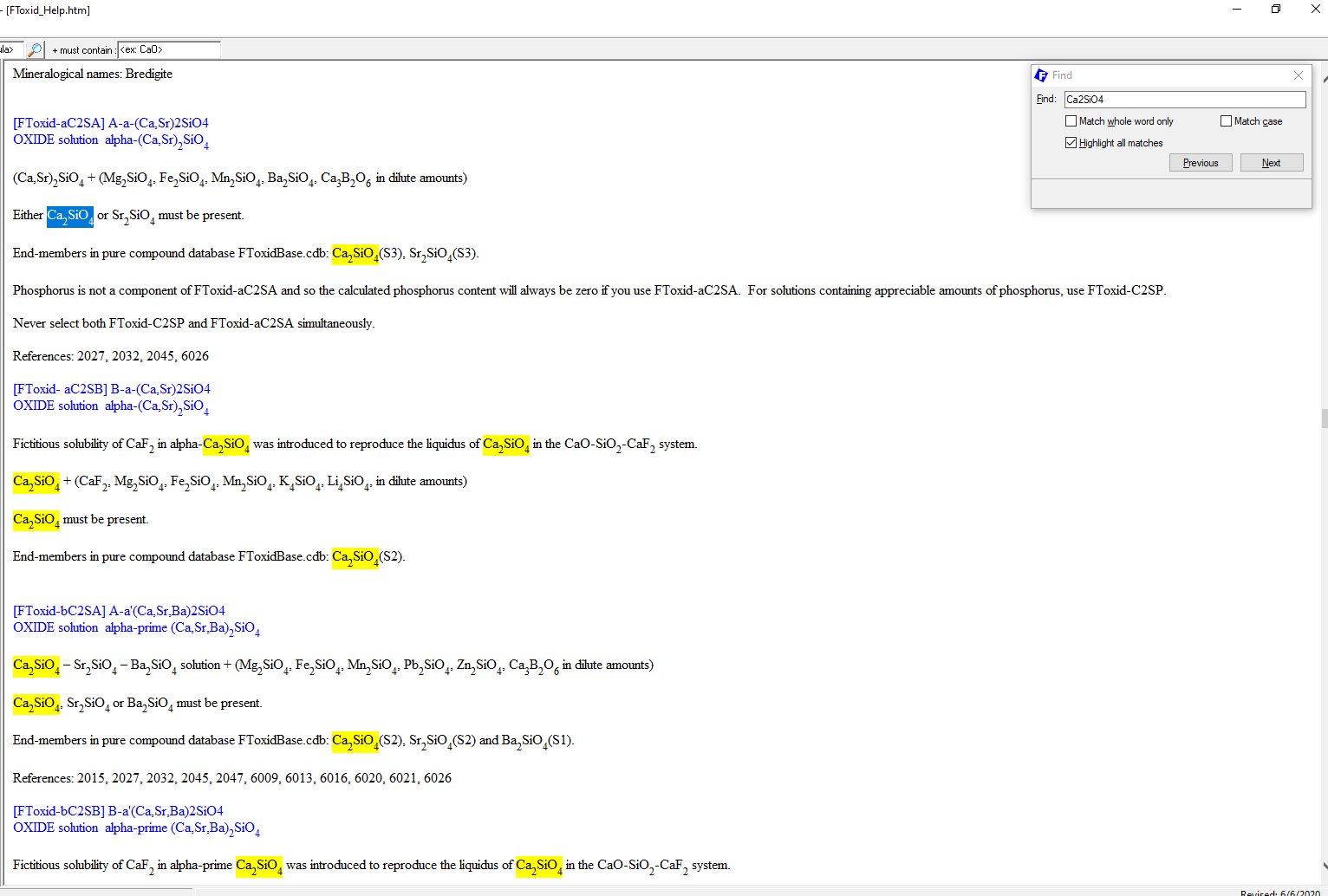
Figure 11
Finding where a species is contained inside a database can be very useful in order to properly define whether a certain solution phase should be included in an equilibrium calculation or not. There are situations when, including the same species twice, once as a pure substance and once as a solution’s constituent, may lead to convergence problems in the calculation. In these situations, as a rule, one should select only the solution phase to be considered in the calculation.
