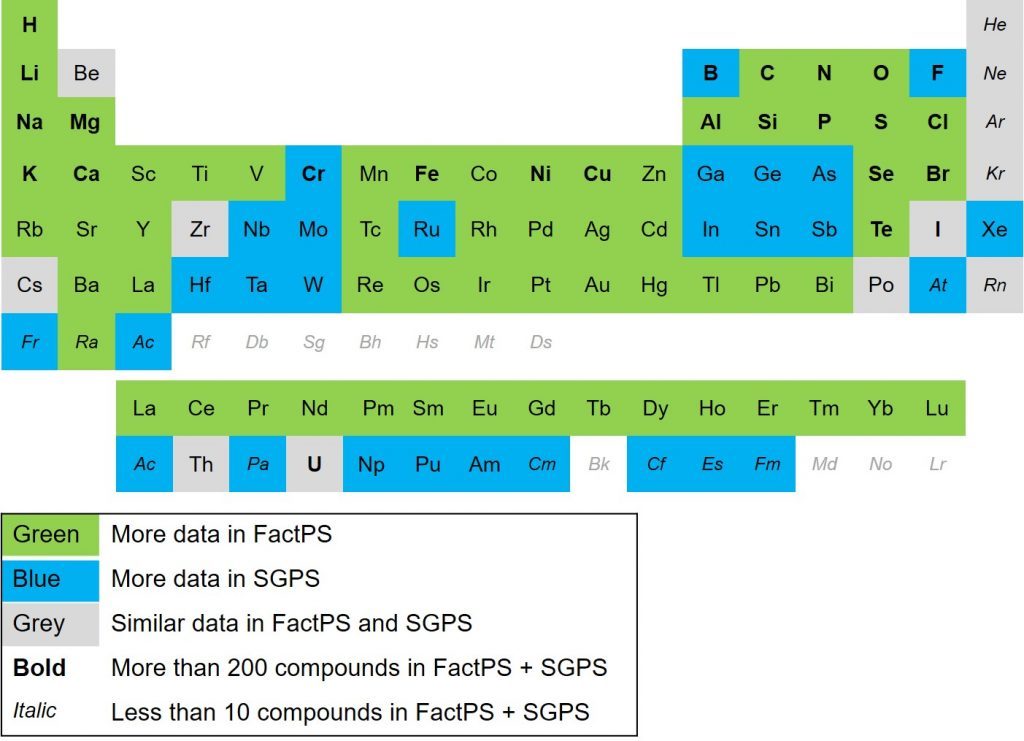
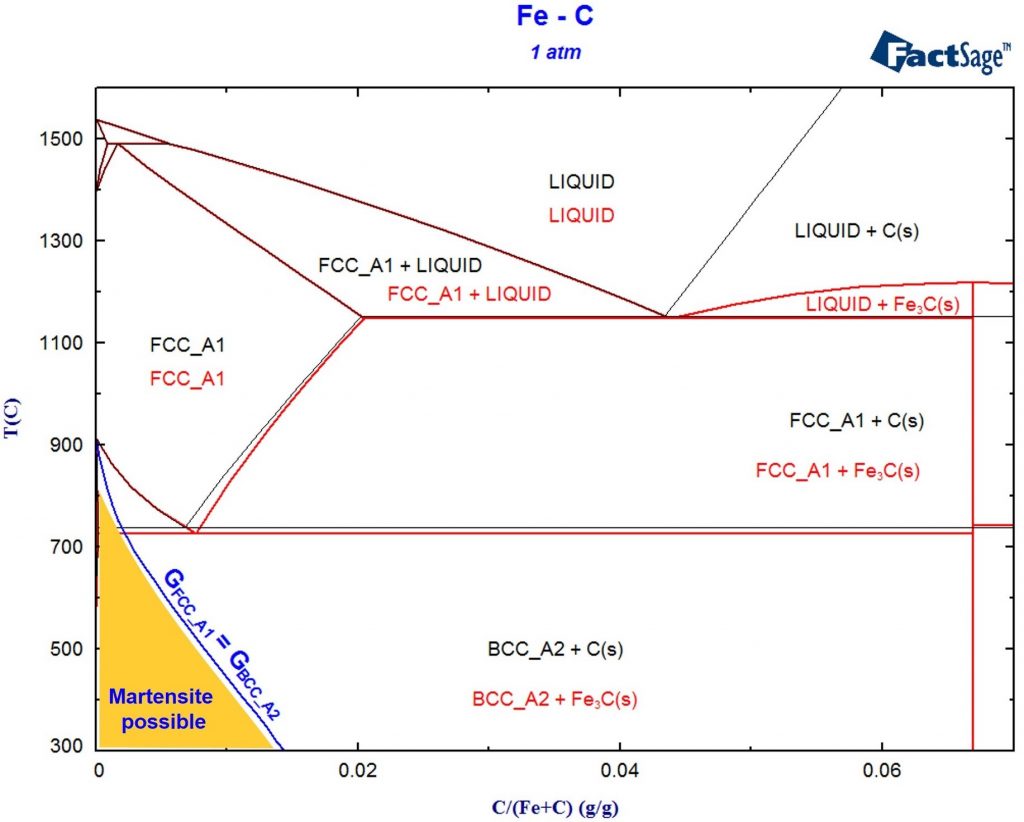
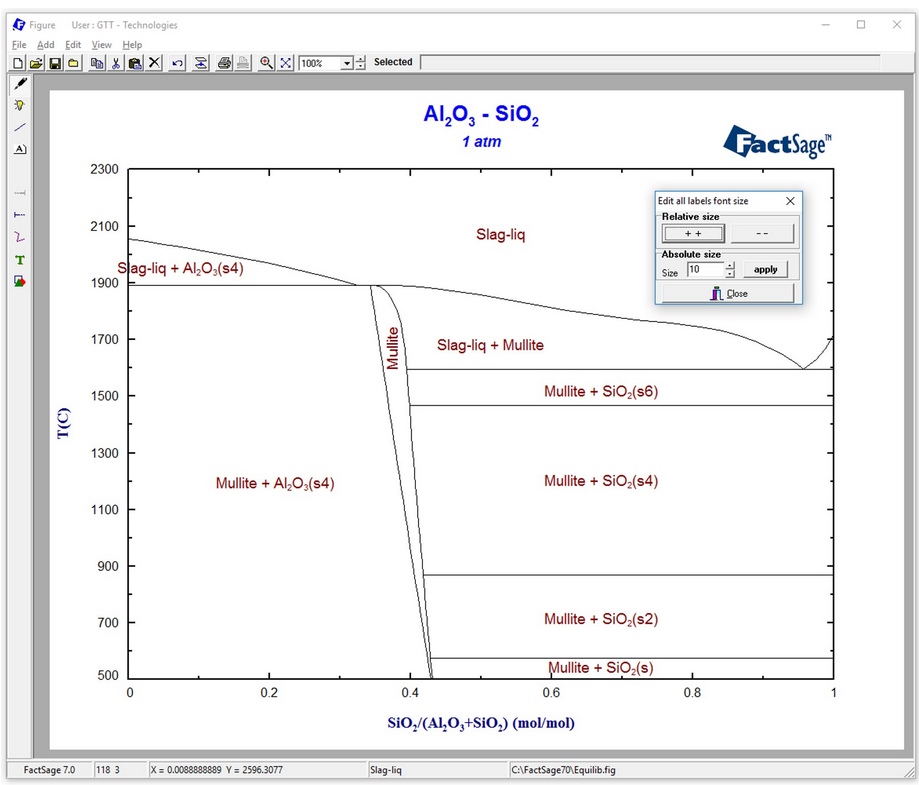
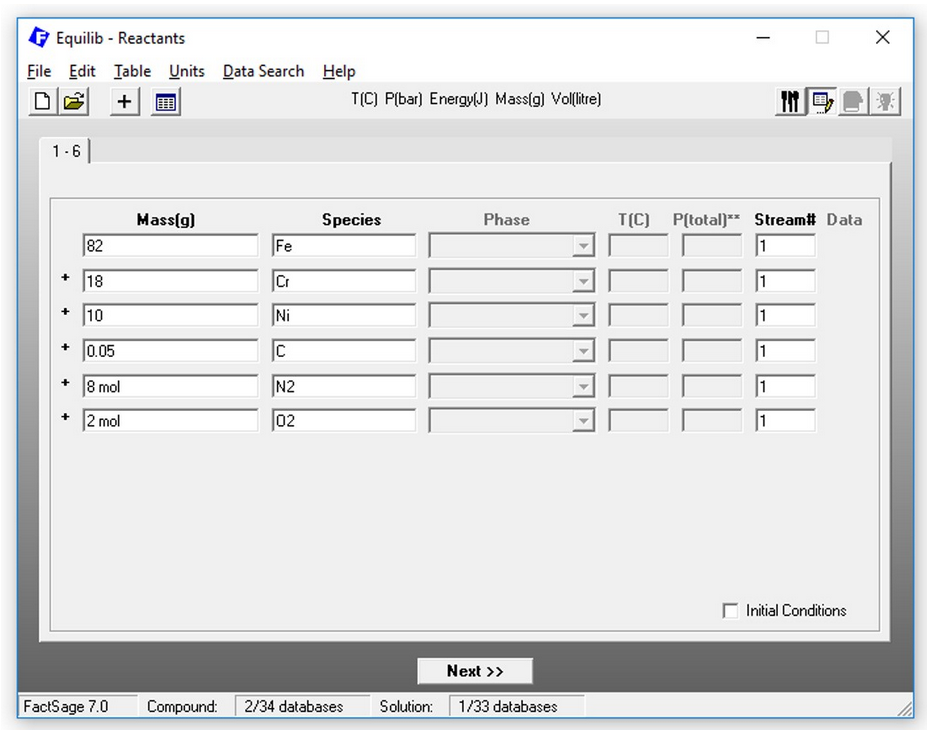
Minimization of copper losses to the converter slag through a boron oxide addition
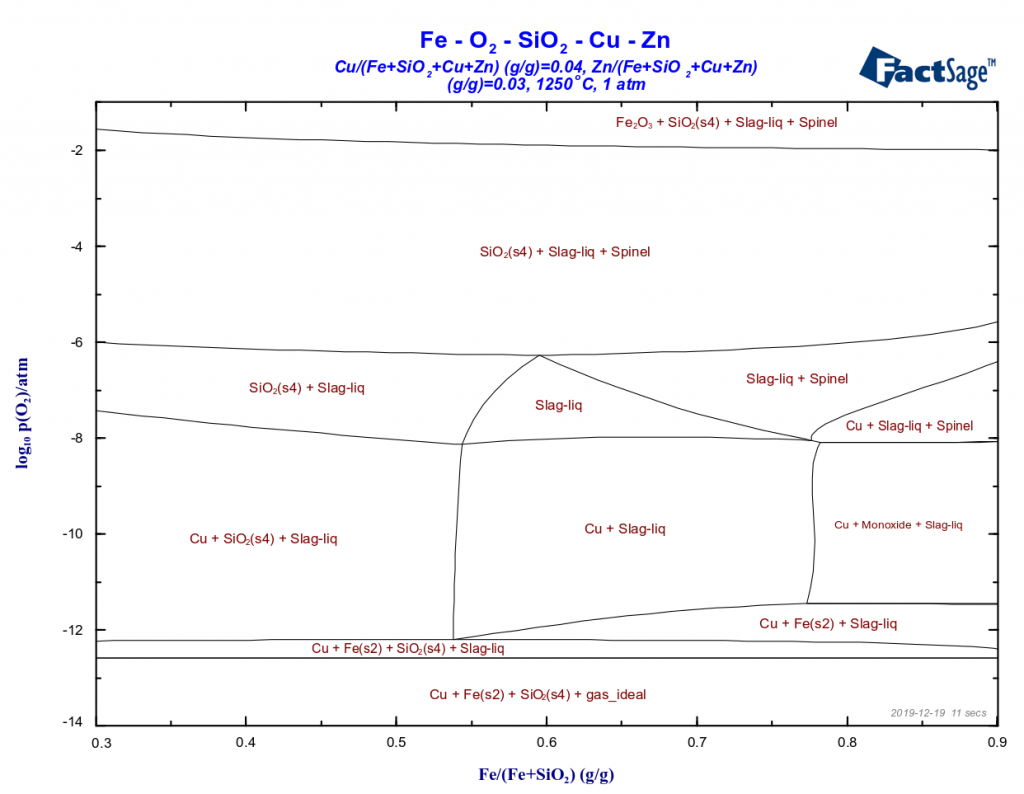
The loss of copper in the converting process through its incorporation to the converter slag represents a major problem in the copper metallurgy. There are two important mechanisms for this phenomenon: the physicochemical and the mechanical one. The mechanical mechanism, i.e. the mechanical entrapment of matte or of metallic copper inside the slag, is by […]
Minimization of copper losses to the converter slag through a boron oxide addition Read More »